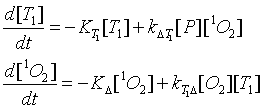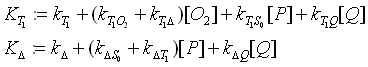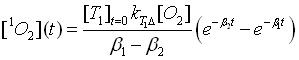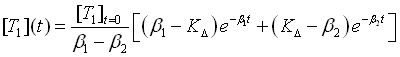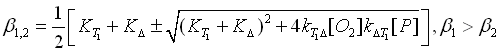|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DR. JÜRGEN BAIER |
| Time-resolved investigations of singlet oxygen
luminescence in vitro and in vivo
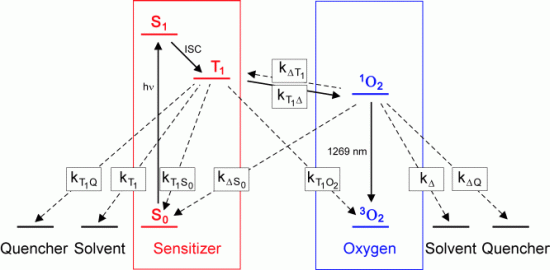
Singlet oxygen Singlet oxygen ( Photosensitized generation of singlet oxygen The energy level diagram for the excitation of singlet oxygen by energy transfer from the triplet T1 state of the photosensitizer and the deactivation of singlet oxygen and the T1 state is given by the following figure. It contains the singlet ground state S0 of the photosensitizer,
its first excited singlet state S1 and its first excited triplet state
T1. The scheme contains also the ground state of oxygen 3O2 and the first
excited singlet state of oxygen, (1O2). Additionally, the figure shows
the ground state of the solvent and the ground state of a possible quencher.
Vibronic states are not included. The relaxation rates kj and the rate
constants kij describe the frequency of the occurrence of the relaxation.
The system of the differential equations can be considered a harmonic oscillator and the solutions of the system are given by
for the time dependence of the population of singlet oxygen and
for the time dependence of the population of the triplet T1 state of the photosensitizer. Here [T1]t=0 is the initial population density of the triplet state T1 of the photosensitizer. The rates beta1 and beta2 are defined by
The experimental luminescence signal at 1270 nm can be
described by Equation [1O2](t). The rates beta1 and beta2 correspond the
rise rate and the decay rate of the luminescence signal, respectively.
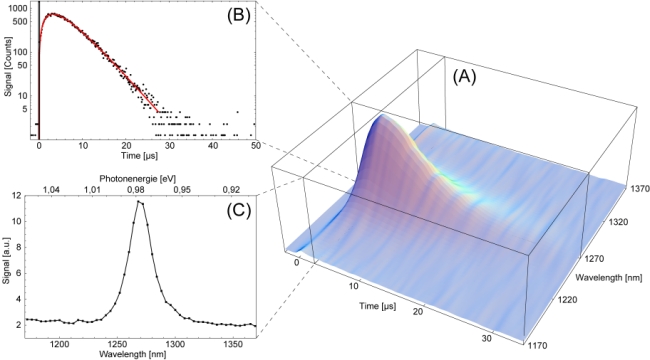
Luminescence of singlet oxygen In the Figure above the time independence luminescence of singlet oxygen was measured at different wavelengths from 1170 nm to 1370 nm. The singlet oxygen was generated by 50 µM Riboflavin
in H2O. The irradiation wavelength was 355 nm and solvent was air saturated.
All measurements were added to a three-dimensional plot in Figure A. The
typical measured logarithmic signal at 1270 nm is shown in Figure B. At
0 µs the laser pulse is shown and the solid line represent the theoretical
fit according [1O2](t). The signals at 1270 nm were taken to determine
the raise (beta1) and decay rate (beta2). In this case the signal raises
with 3.3±0.5 µs and decays with 3.2±0.5 µs. In
Figure C the wavelength scan by summing up the luminescence signals at
each wavelength is shown. The maximum at 1270 nm which represent photon
energy of 0.976eV is a unique evidence for the luminescence of singlet
oxygen.
Publications Theoretical and experimental analysis of the luminescence
signal of singlet oxygen for different photosensitizers
The role of singlet oxygen and oxygen concentration in
photodynamic inactivation of bacteria
Direct Detection of Singlet Oxygen Generated by UVA Irradiation
in Human Cells and Skin
Singlet oxygen generation by UVA light exposure of endogenous
photosensitizers
Time-Resolved Investigations of Singlet Oxygen Luminescence
in Water, in Phosphatidylcholine, and in Aqueous Suspensions of Phosphatidylcholine
or HT29 Cells
Singlet oxygen generation by 9-acetoxy-2,7,12,17-tetrakis-(beta-methoxyethyl)-porphycene
(ATMPn) in solution
Singlet Oxygen Generation by 8-Methoxypsoralen in Deuterium
Oxide: Relaxation Rate Constants and Dependence of the Generation Efficacy
on the Oxygen Partial Pressure
Bidirectional energy transfer between the triplet T1 state
of photofrin and singlet oxygen in deuterium oxide
|